MDT Stock Recent News
MDT LATEST HEADLINES
The magic of dividends is that you don't need to do anything to earn them aside from holding the stocks that pay them. Someone who accumulates enough dividend stocks could live off their portfolio's passive income stream and never have to work another day in their life.
GALWAY, Ireland , June 23, 2025 /PRNewswire/ -- Medtronic plc (NYSE: MDT), a global leader in healthcare technology, today announced that its Board of Directors appointed Dr. Joon Lee, CEO at Emory Healthcare, Inc., to the Board as an independent director, effective June 18, 2025. Dr. Lee will serve on the Science and Technology Committee and Compensation and Talent Committee of the Board.
The healthcare sector isn't exactly known for offering huge yields, with Health Care Select Sector SPDR (NYSEMKT: XLV) offering a yield of just 1.7%. If dividend investors take some time to dig into the sector, however, they can do much better.
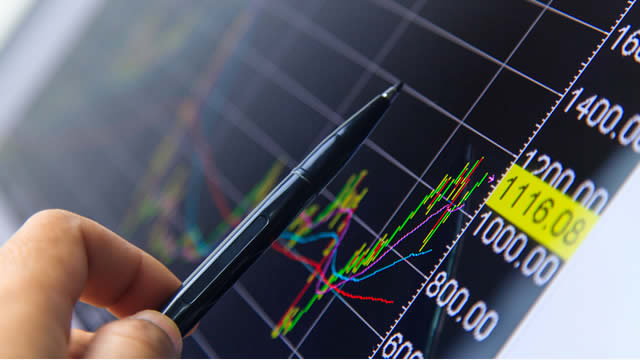
Medtronic (MDT) reported earnings 30 days ago. What's next for the stock?
I focus on companies with consistent dividend growth, using a blend of the U.S. Dividend Champions list and NASDAQ data for my selections. This week's highlighted group averages a 5.9% dividend increase and a 19.5-year streak, but none outperformed the SCHD ETF over the past decade. While SCHD remains my benchmark for dividend growth and total return, I look for individual stocks that can deliver significant alpha over it.
Medtronic remains a dependable dividend aristocrat, but its long-term total shareholder returns lag significantly behind peers like Johnson & Johnson and Stryker. Despite a solid 2025 performance, the stock's recent gains stem from a low base, and it still trades well below its 2021 highs. Earnings growth is steady but uninspiring, with margin pressure and tariff risks highlighting operational challenges versus higher-quality competitors.
Medtronic plc MDT announced on May 21 its plans to separate its Diabetes business into a separate, publicly traded company. The separation will occur via a two step transaction – an initial minority IPO carve-out followed by a split-off of the remaining stake.
The S&P 500 index (^GSPC 0.38%) is offering a minuscule 1.2% average yield today. That's not much to support a dividend-focused investor's income needs and speaks to a market that is richly valued.
Medtronic (MDT 0.54%) is a large medical device maker, but its stock hasn't performed particularly well of late. After hitting a peak in 2021, the stock fell nearly 50% and has since moved roughly sideways.
Volatility is a part of life for long-term investors. The reasons the market goes up or down change, but the constant is that high-quality stocks, businesses that increasingly make more money over time, will reward investors if you wait long enough.