MESO Stock Recent News
MESO LATEST HEADLINES
Does Mesoblast Limited (MESO) have what it takes to be a top stock pick for momentum investors? Let's find out.
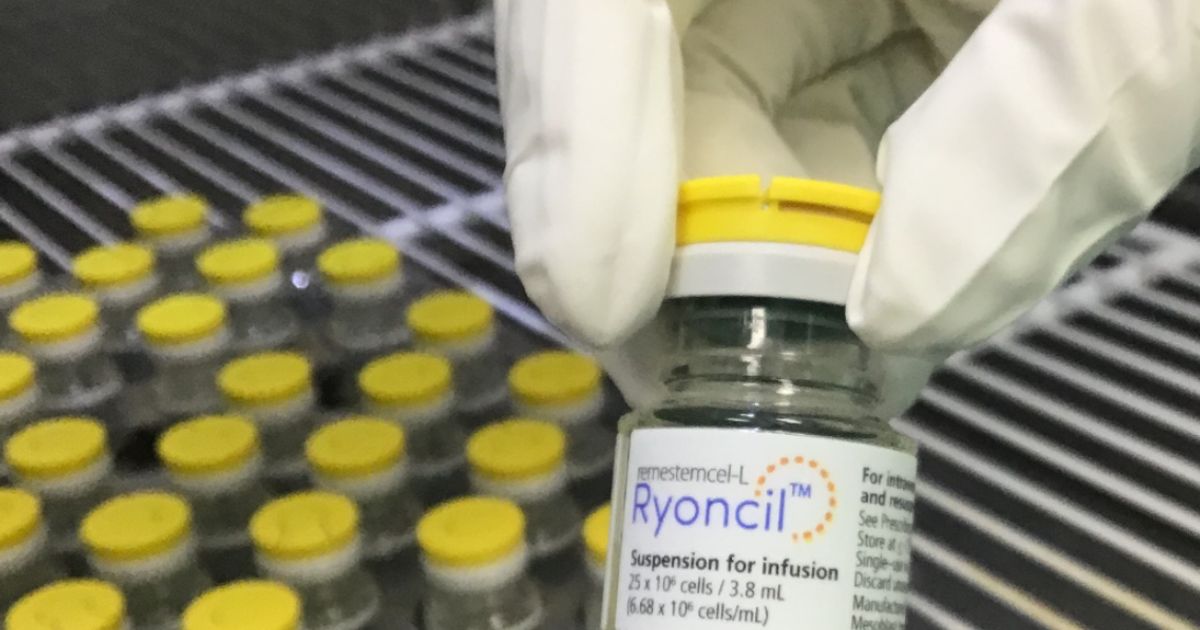
Shares in Mesoblast Ltd (NASDAQ:MESO, ASX:MSB) surged by nearly 38% following Friday’s successful commercial launch of its FDA-approved treatment, Ryoncil®, for steroid-refractory acute graft-versus-host disease (SR-aGvHD) in children. The Melbourne-based biotechnology company reported a strong start to the first quarter post-launch, with gross revenue of US$13.2 million for the period ending June 30, 2025. The stock gained almost 35% over the week to reach $2.41, buoyed by the positive update. However, the momentum appears to be tapering off this week, with shares falling by 3.7% today to A$2.32. The release of Ryoncil® marked a significant milestone for Mesoblast, as it is the first and only FDA-approved mesenchymal stromal cell (MSC) product for the treatment of SR-aGvHD in pediatric patients. Mesoblast’s solid financial standing remains a key asset, with US$162 million (A$247 million) in cash on hand at the end of the quarter, positioning the company for continued expansion.
NEW YORK, July 17, 2025 (GLOBE NEWSWIRE) -- Mesoblast Limited (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today announced gross revenue from sales of Ryoncil® (remestemcel-L-rknd) through the first quarter post product launch. Ryoncil® is the first and only FDA approved mesenchymal stromal cell (MSC) product in the Unites States and became commercially available for purchase on March 28, 2025, within one quarter of receiving FDA approval for treatment of steroid-refractory acute graft-versus-host disease (SR-aGvHD) in children.
On Monday, Mesoblast Ltd. MESO announced alignment with the U.S. Food and Drug Administration (FDA) on items required for filing a Biologics License Application (BLA) for Revascor (rexlemestrocel-L) for ischemic heart failure with reduced ejection fraction (HFrEF) and inflammation.
NEW YORK, June 30, 2025 (GLOBE NEWSWIRE) -- Mesoblast (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today announced alignment with the United States Food and Drug Administration (FDA) on items required for filing a Biologics License Application (BLA) for Revascor® (rexlemestrocel-L) in the treatment of patients with ischemic heart failure with reduced ejection fraction (HFrEF) and inflammation.
NEW YORK, June 11, 2025 (GLOBE NEWSWIRE) -- Mesoblast (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today provided an update on continued momentum with United States Food and Drug Administration (FDA) regarding both accelerated approval pathway for Revascor® (rexlemestrocel-L) in the treatment of patients with ischemic chronic heart failure with reduced ejection fraction (HFrEF) and inflammation, and label extension for Ryoncil® (remestemcel-L-rknd) in adults with steroid refractory acute graft versus host disease (SR-aGvHD).
NEW YORK, May 14, 2025 (GLOBE NEWSWIRE) -- Mesoblast (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today announced that it has received seven years of orphan-drug exclusive approval from U.S. Food and Drug Administration (FDA) for Ryoncil® (remestemcel-L) for treatment of steroid-refractory acute graft versus host disease (SR-aGvHD) in pediatric patients 2 months of age and older.
MESO's Off-the-shelf MSC/MPC platform treats severe inflammatory diseases without donor–recipient matching. This alleviates the typical need for immunosuppression. The FDA recently approved Ryoncil, which achieved as much as 70% response in pediatric SR-aGVHD patients. And it has adult SR-aGVHD and Crohn's Phase 3 trials underway. MESO's Revascor is also in trials for chronic low back pain and heart failure, and its accelerated BLA could position it as another revenue vertical in the next couple of.
NEW YORK, April 29, 2025 (GLOBE NEWSWIRE) -- Mesoblast Limited (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today provided highlights of its recent activities for the third quarter ended March 31, 2025.
NEW YORK, April 28, 2025 (GLOBE NEWSWIRE) -- Mesoblast (Nasdaq:MESO; ASX:MSB), global leader in allogeneic cellular medicines for inflammatory diseases, today announced that it has appointed Lyn Cobley to its Board of Directors. Ms. Cobley has been a global leader in the financial services industry with over 30 years' experience in senior positions at international and domestic banks. Ms. Cobley has served as CEO of Westpac Institutional Bank, Group Treasurer of Commonwealth Bank of Australia, and held senior roles at Barclays Capital and Citibank Limited.