REGN Stock Recent News
REGN LATEST HEADLINES
NEW YORK, Sept. 09, 2025 (GLOBE NEWSWIRE) -- Bragar Eagel & Squire, P.C.
Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN ) Morgan Stanley 23rd Annual Global Healthcare Conference September 8, 2025 7:00 AM EDT Company Participants Christopher Fenimore - Executive VP of Finance & CFO Leonard Schleifer - Co-Founder, President, CEO & Co-Chairman Marion McCourt - Executive Vice President of Commercial Conference Call Participants Terence Flynn - Morgan Stanley, Research Division Presentation Terence Flynn Equity Analyst Great. Good morning, everybody.
TARRYTOWN, N.Y., Sept. 04, 2025 (GLOBE NEWSWIRE) -- Regeneron Pharmaceuticals, Inc. (NASDAQ: REGN) announced today that it will now present at the Morgan Stanley 23rd Annual Global Healthcare Conference at 7:00 a.m. ET on Monday, September 8, 2025.
Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN ) Wells Fargo 20th Annual Healthcare Conference 2025 September 4, 2025 9:30 AM EDT Company Participants Ryan Crowe - Senior Vice President of Investor Relations & Strategic Analysis Marion McCourt - Executive Vice President of Commercial Conference Call Participants Mohit Bansal - Wells Fargo Securities, LLC, Research Division Presentation Mohit Bansal Senior Equity Analyst Awesome. Thank you very much for joining us today.
Regeneron Pharmaceuticals, Inc. (NASDAQ:REGN ) Cantor Global Healthcare Conference 2025 September 3, 2025 1:35 PM EDT Company Participants Ryan Crowe - Senior Vice President of Investor Relations & Strategic Analysis Christopher Fenimore - Executive VP of Finance & CFO Conference Call Participants Carter Gould - Cantor Fitzgerald & Co., Research Division Presentation Carter Gould Research Analyst All right. Good afternoon.
Regeneron Pharmaceuticals, Inc. shares have lost >50% of their value over the past 12 months. Regeneron remains a powerhouse pharma however, despite threats to its key drug Eylea from generics and longer acting therapies. Regeneron also co-markets and sells Dupixent, whose revenues exceeded >$4bn last quarter, with Regeneron earning >$1.4bn.
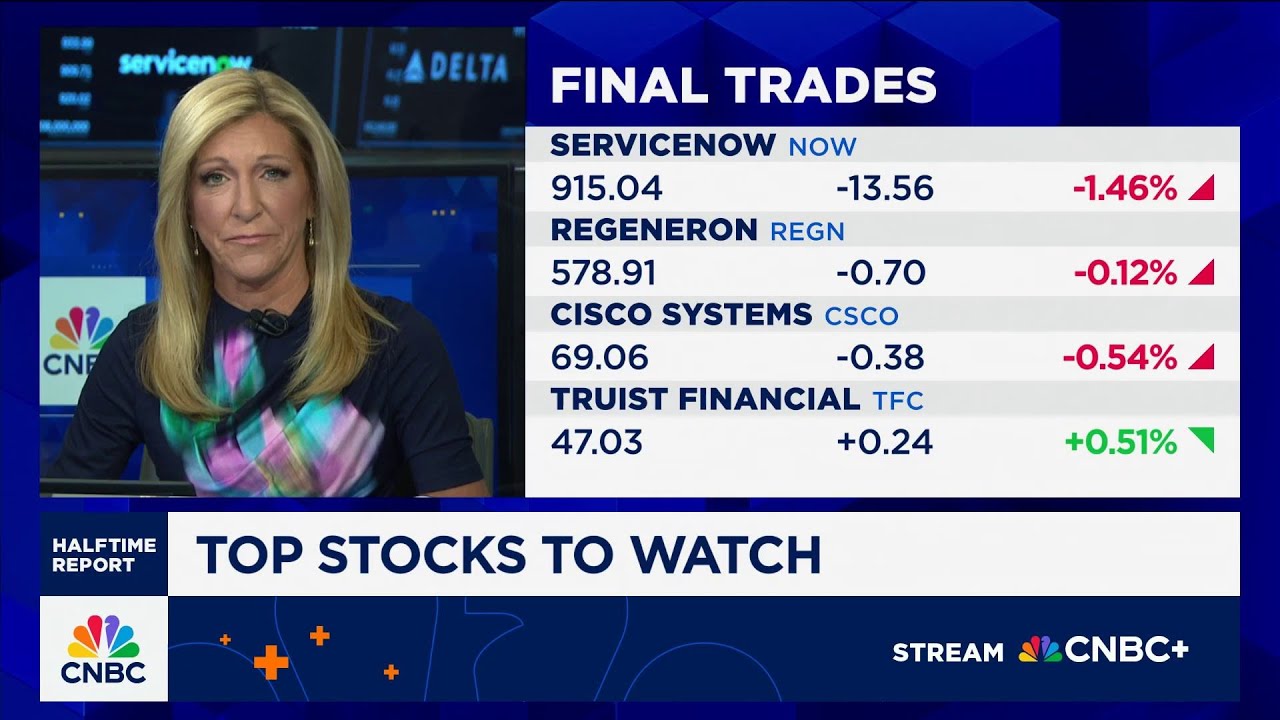
The Investment Committee give you their top stocks to watch for the second half.
Regeneron Pharmaceuticals said on Tuesday its experimental therapy for treating patients with a rare immune disorder has met the main goal of a late-stage study.
Viking Therapeutics (VKTX 1.32%) and Regeneron Pharmaceuticals (REGN -1.45%) have faced challenges this year that have sunk their stock prices. The former is down by 37% this year, while the latter has declined 17%.
The FDA extends action dates for Regeneron's Eylea HD filings following inspection findings at Catalent Indiana. Nonetheless, Eylea HD remains available through vial administration.