SNY Stock Recent News
SNY LATEST HEADLINES
Sanofi's gene therapy SAR402663 for wet AMD earns FDA fast track status, aiming to speed up development and reduce treatment burden.
Sanofi's SAR402663 earns fast track designation in the US for neovascular age-related macular degeneration Designation earned for a one-time intravitreal gene therapy with the potential to eliminate treatment burden for people living with neovascular age-related macular degeneration Neovascular or “wet” age-related macular degeneration can lead to significant vision loss and affects more than one million people in the US Paris, September 11, 2025. The US Food and Drug Administration (FDA) has granted fast track designation to SAR402663, an investigational one-time intravitreal gene therapy for the treatment of neovascular age-related macular degeneration (AMD).
Mandy Moore joins Elaine Welteroth, Gaby Dalkin, Katya Echazarreta, and Shawn Johnson East to share their personal respiratory syncytial virus (RSV) stories and why they chose BEYFORTUS to help protect their babies from serious RSV lung infection They join the millions of parents in the US who have chosen BEYFORTUS to help protect their babies from RSV, the number one reason babies under 1 are hospitalized MORRISTOWN, N.J. , Sept. 10, 2025 /PRNewswire/ -- Sanofi is partnering with celebrity moms who chose to immunize their babies with BEYFORTUS® (nirsevimab-alip) to help educate families about the importance of RSV infant protection.
Tzield approved in China as first disease-modifying therapy for adult and pediatric patients with stage 2 type 1 diabetes
Sanofi (NASDAQ:SNY ) Morgan Stanley 23rd Annual Global Healthcare Conference September 9, 2025 1:05 PM EDT Company Participants Paul Hudson - CEO & Director Conference Call Participants Sarita Kapila - Morgan Stanley, Research Division Presentation Sarita Kapila Equity Analyst Okay. Perfect. Let's get started.
Sanofi shares fall despite phase III success for eczema drug candidate, amlitelimab, showing strong efficacy and safety in patients.
French drugmaker Sanofi SA SNY stock fell sharply Thursday after late-stage trial results for amlitelimab, its potential successor to blockbuster eczema drug Dupixent, failed to match investor expectations, raising fresh doubts about the company's ability to sustain its dermatology franchise once patent protections expire.
Sanofi shares plunged at the market open Thursday as results from a late-stage trial of its experimental drug to treat skin conditions disappointed investors.
Shares in French drugmaker Sanofi fell more than 9% on Thursday after late-stage trial data for its experimental inflammatory disease drug amlitelimab fell short of Wall Street expectations.
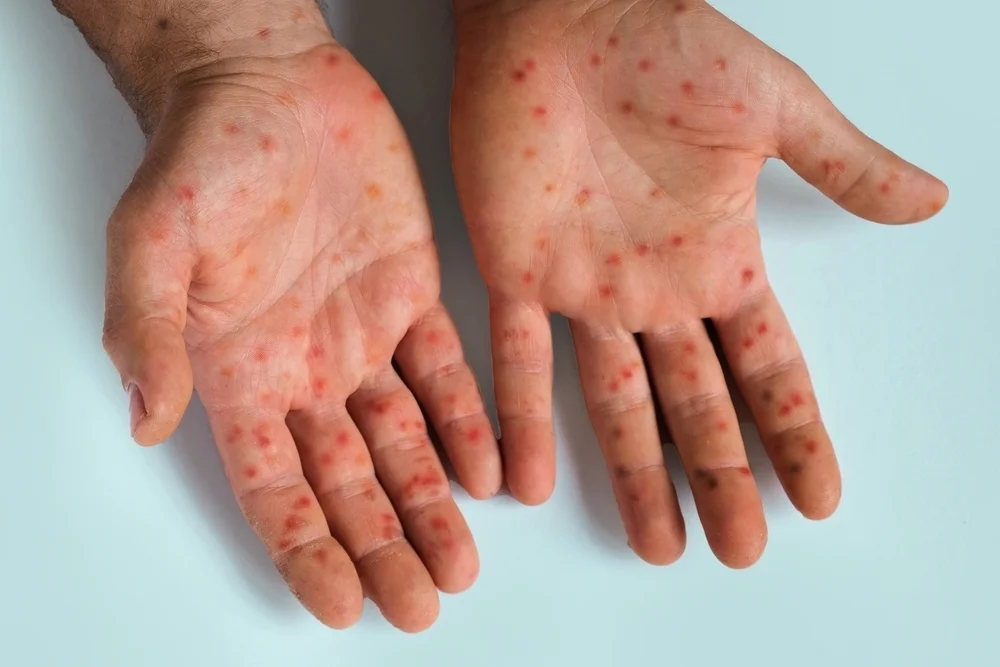
Amlitelimab showed efficacy in skin clearance and disease severity compared to placebo in a phase 3 study with adolescent and adults with atopic dermatitis.